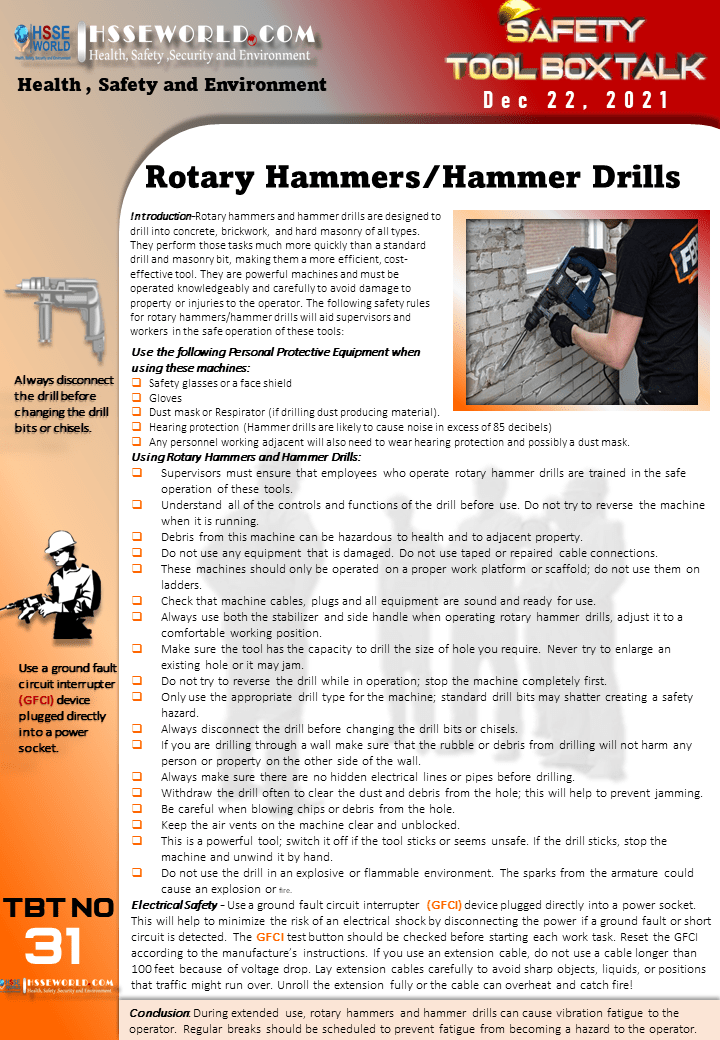
There have been numerous recorded incidents where failings by operators have been the major contributing cause of major accidents. Provision of clear, concise and accurate operating procedures is the most effective measure to prevent, control and mitigate such events.
Operating procedures should clearly lay down instructions for operation of process plant that take into consideration COSHH, manual handling, permit to work, PPE Regulations, quality, HAZOP, and SHE requirements. The procedure should represent a definition of good or best practice that should be adhered to at all times. Process operatives should be provided with guidance concerning the required operating philosophy to ensure that they comply with procedural requirements.
Adequate training should be provided to ensure that operators are fully conversant with written procedures. This is covered in the Technical Measures Document on Training.
It is important that operating procedures should always reflect plant practice, and vice versa.

Provision of Comprehensive Written Operating procedures
Comprehensive written operating procedures should be generated where applicable that address:
- Standard operating procedures and operating philosophy;
- Abnormal operating procedures;
- Temporary operating procedures;
- Plant trials;
- Emergency operating procedures;
- Commissioning;
- Plant Start-up;
- Plant Shut-down;
- Bulk loading and unloading;
- Process change;
- Plant change.
These procedures should cover the following:
- Material safety data (COSHH);
- Plant operatives should have an awareness and understanding of material safety data for raw materials, intermediates, products and effluent / waste;
- Control measures and personal protective equipment;
- Location of plant where process to be undertaken;
- Roles and responsibilities of individuals involved in plant operations;
- Plant fit for purpose;
- The condition of main process plant and equipment (clean, empty etc. as appropriate) should be established as being fit for purpose;
- The condition of ancillary process plant and equipment (clean, empty etc. as appropriate);
- Plant correctly set-up for processing;
- Process monitoring and recording;
- Monitoring and recording of key process parameters (temperature, pressure etc.) in plant logs;
- Quality;
- Sampling of raw materials, intermediates, products and effluent/waste;
- Packaging of final product.
Operating procedures should be controlled documents, generally covered under the company’s quality system and thus kept fully up to date. Any changes should be fully controlled and documented and should be subject to company change procedures (see Technical Measures Document on Plant Modification / Change Procedures). Standard operating procedures may be revised for the following reasons:
- Introduction of new equipment into the process;
- Introduction of new chemicals into the process;
- Significant change to process, task, personnel or equipment covered by the procedure;
- Plant trials have been successful and need to be incorporated into standard operating procedures.